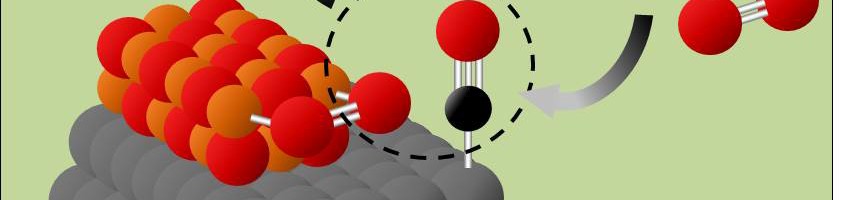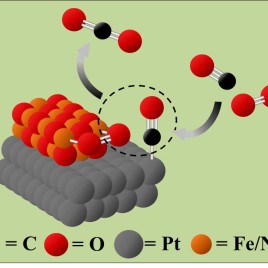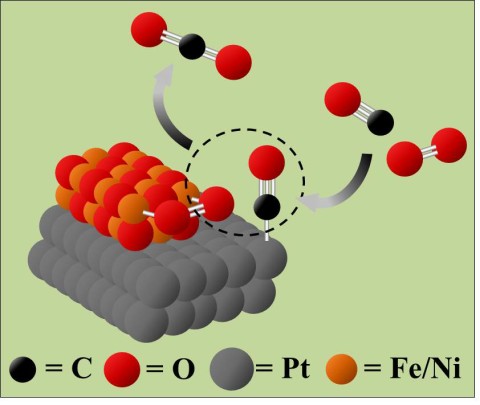
Catalysts made from platinum (Pt), iron (Fe), and nickel (Ni) speed up the reaction between toxic carbon monoxide (CO) and oxygen (O2) to make more benign carbon dioxide (CO2). (Credit: Paul Duchesne)
Removing toxic carbon monoxide (CO) from car exhaust and other pollution sources could soon be cheaper and more efficient, thanks to a new type of nanoparticle catalyst. Catalytic converters that turn CO into benign CO2 rely on expensive metals like platinum to speed up the chemical reaction. The new nanoparticles combine platinum with cheaper iron oxide and nickel oxides, yet they achieve even better catalytic activity. This is because their unique structural design bring together CO and O2 molecule and makes it easier for them to react, even at room temperature.
Original research paper published in the journal Science on May 1, 2014.
Names and affiliations of selected authors