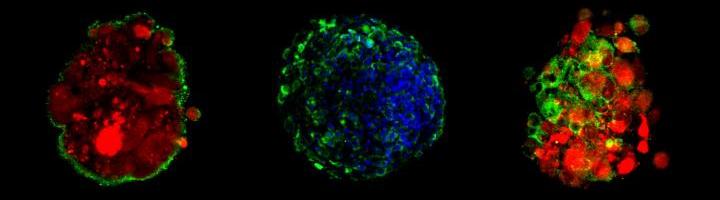
Blood-brain-barrier spheroids present high level of efflux pump (left: green) and tight junctions (center and right: green) on the surface of each spheroid to keep foreign molecules out.
(Image courtesy of Choi-Fong Cho, Brigham and Women’s Hospital)
Selective membrane that protects the central nervous system from circulating blood, commonly known as the blood-brain barrier, has been notoriously hard to penetrate — for a good reason, as it protects cells of the central nervous system from contamination. But breaching this barrier is also essential for delivering medicine to treat illnesses affecting the CNS and certain cancers. A newly designed model of the membrane, described in a recent paper, closely mimics the structure of the barrier in the human body — unlike previous models, which have been either animal-based or lab-grown. The current model grows different kinds of brain cells—endothelial cells, pericytes and astrocytes—together, allowing them to spontaneously form multicellular spheroids, which resemble the structure of the human blood-brain membrane. This model can help better predict the efficacy of drug delivery into the human central nervous system.
Authors:
Choi-Fong Cho, Justin M. Wolfe, Colin M. Fadzen, David Calligaris, Kalvis Hornburg, E. Antonio Chiocca, Nathalie Y. R. Agar, Bradley L. Pentelute & Sean E. Lawler
Canadian lead author:
Choi-Fong Cho, Brigham and Women’s Hospital, Department of Neurosurgery, Harvard Medical School, Email: ccho6@partners.org
Original paper published in Nature Communications on June 6, 2017.